Hydrogen – an interesting source of energy
Sattec develops seals for future applications
Hydrogen can make a significant contribution to climate protection – as a fuel for cars, as a raw material for industry or as a fuel for heating systems. As a versatile energy carrier, it can be used in all sectors and therefore plays a key role in the energy transition. As part of the European Green Deal, the conditions for the so-called green transition in the gas sector have also been created and the use of clean gases, such as hydrogen, is being promoted [1].
The special properties of this gas have made it one of the most interesting new energy sources, and its economic importance in the coming decades could even be comparable to that of oil or natural gas, but without the known negative consequences for the environment.
Properties of hydrogen
Hydrogen is the most abundant element in the universe. For example, 75 % of the mass of the sun consists of hydrogen [2]. Hydrogen is also abundant on Earth, but not in its ‘pure’ form as H2. As soon as it is released, its atoms immediately find another element with which to combine to form a molecule: For example, two hydrogen atoms combine with an oxygen atom to form water (H2O), four hydrogen atoms combine with a carbon atom to form methane (CH4), and so on for an infinite number of combinations until ever more complex molecules are formed. Due to its small size – hydrogen has atomic number 1 and is in the first period of the periodic table – hydrogen also has the highest diffusion capacity. This presents technical challenges in the transport and storage of hydrogen.
Hydrogen production
Hydrogen is also produced in different ways because of its different forms of bonding. To make it easier to distinguish, different colours are assigned to the different types of hydrogen production. There are green, turquoise, grey and blue hydrogen.
- Green hydrogen is produced by electrolysis (splitting water into oxygen and hydrogen). Renewable energy sources such as wind, water or solar power provide the electricity required (powerto- gas technology). This makes the production of green hydrogen CO2-neutral, although it is still associated with high energy consumption. In Germany, there are already 40 plants for the production of green hydrogen (so-called electrolysers) [3].
- Turquoise hydrogen is the product of methane pyrolysis. In this process, the methane in natural gas is split into hydrogen and solid carbon. Solid carbon is a granular material that can be safely stored in old mining tunnels, for example, and reused later. This means that no CO2 is released into the atmosphere. If the energy required for methane pyrolysis comes from renewable sources, the production of turquoise hydrogen is climate-neutral.
- Grey hydrogen is produced by steam reforming fossil fuels such as natural gas, coal or oil. This produces CO2 as a waste product, which is released into the atmosphere. Grey hydrogen is therefore not climate neutral.
- Like grey hydrogen, blue hydrogen is produced by steam reforming, but the CO2 produced is stored underground (CCS technology – carbon capture and storage). It is therefore not released into the atmosphere and is also climate neutral.
A more production-related description of the types of hydrogen can be found in the Communication of the European Commission [4]. Green hydrogen as defined in [3] is roughly equivalent to “renewable hydrogen” as defined in [4].
Renewable hydrogen is the only hydrogen that combines two important advantages:
- It is climate-neutral and its production is based on renewable energy sources rather than fossil fuels.
- Its use produces “zero CO2 emissions”; only water vapour is released into the air during combustion.
This completely “green” process is a key element in bringing all resources into line with decarbonisation plans.
Hydrogen strategies
As early as 2020, the European Commission published “A Hydrogen Strategy for a Climate Neutral Europe” in a Communication of 8 July 2020. The EU is prioritising the development of renewable hydrogen, mainly produced from wind and solar energy. Renewable hydrogen is the option that is most compatible with the EU’s long-term goal of climate neutrality and zero emissions, as well as an integrated energy system [4] that aims to become “carbon neutral” by 2050.
As a result of the energy crisis, the search for renewable energy sources and the objectives to reduce CO2 emissions, all European countries are developing policies and technologies to promote the use of hydrogen as an alternative energy source to natural gas/oil. For example, the following are being developed
- Gas microturbines for electricity generation that run on a mixture of methane and hydrogen,
- Car engines running on a mixture of hydrogen and methane, using the same engine design and ensuring the same performance and efficiency, with lower CO2 emissions compared to natural gas and diesel (-10 % compared to natural gas and -20 % compared to diesel),
- Hydrogen boilers for domestic use that can produce electricity, hot water and heating without connection to external energy sources and without releasing exhaust gases or waste into the environment, resulting in 0 % CO2 emissions.
Characteristics create challenges
There are, of course, technical challenges associated with the use of hydrogen. Relevant characteristics are
- The high tendency to escape from systems (diffusion) due to the small size of its molecule, which is relevant to the transport and storage of hydrogen,
- the tendency to interact with materials and weaken them,
- its high flammability, which is much higher than that of natural gas or LPG.
These characteristics pose a number of challenges for the technology, production, transport, distribution and use of hydrogen.
There are a number of hypotheses on how hydrogen can be transported for different end uses:
- Hypothesis 1: Generation and transmission to residential, commercial and industrial end-users. This option eliminates the cost of transporting hydrogen, but at the expense of electricity grids that are already burdened with transporting increasing amounts of renewable energy.
- Hypothesis 2: Deliver hydrogen directly to consumers. This solution has no impact on electricity grids, but requires the upgrading of existing gas pipelines to allow the transport of increasing amounts of hydrogen. Hypothesis 2, using existing
Hypothesis 2, using existing gas networks, could significantly reduce the cost of transporting hydrogen, both in terms of reduced investment in new pipelines and in terms of upgrading electricity grids.
The existing gas distribution networks in Germany have already been assessed for their compatibility with a gradual switch from natural gas to hydrogen. For example, the steel pipelines installed in the German gas network are suitable for transporting hydrogen. They show no differences in their basic suitability for the transport of hydrogen compared to natural gas. Both the operational ageing and the required fracture toughness meet the expectations for decades of safe availability (see [5]).
However, it is not only the walls of gas pipes that need to be analysed and, if necessary, further developed with regard to their suitability for use in future hydrogen networks. The same applies to elastomeric sealing materials and sealings, e.g. for flange connections or in fittings:
- It is estimated that, due to its low density, about three times as much hydrogen can “escape” through a leak path of the same size as methane.
- Due to the small size of its molecule, hydrogen has a higher and faster “permeability” through elastomeric materials such as O-rings, seals, diaphragms, etc. than other gases.
- Leakage must be assessed in a state of the art laboratory and tested to defined standards.
Development of seals for use in hydrogen networks
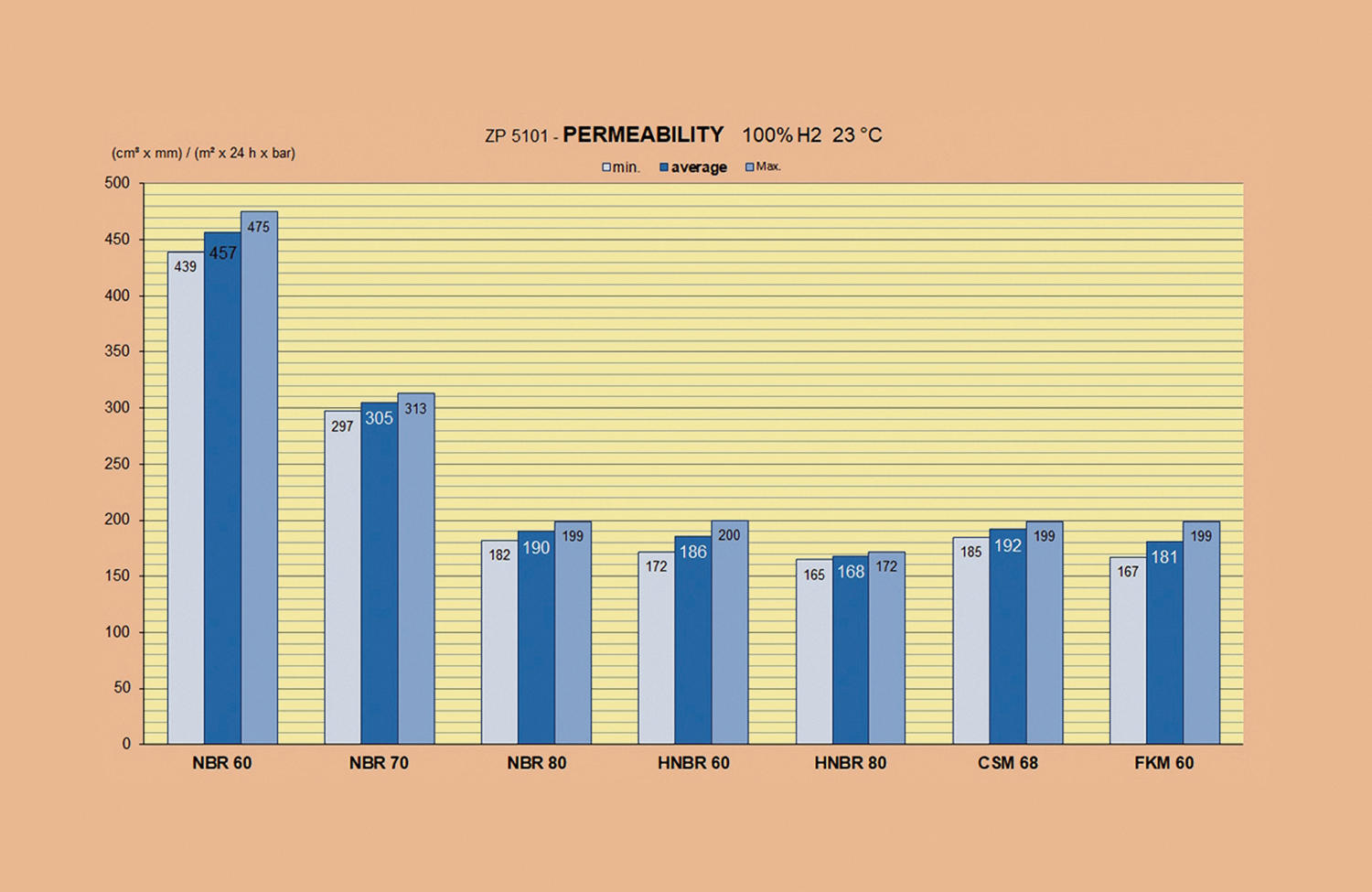
Sattec currently manufactures gas seals with NBR-based compounds that meet the requirements of EN 682 [6] and EN 549 [7]. The company has started a programme to develop compounds for hydrogen applications. This is based on the ZP 5101 certification programme of DVGW CERT GmbH [8]. The subject of this certification programme is the material testing of elastomer materials that have already been DIN-DVGW certified according to EN 549 [7] or EN 682 [6].
ZP 5101 is used to determine hydrogen permeability using a pressure rise method based on ISO 15105-1; this test is used to determine a coefficient to characterise the material. With H2 permeability as a material property, the method presented in ZP 5101 provides an additional technical characteristic value that allows different materials to be compared with respect to hydrogen permeation. The ZP thus supports the selection of elastomer materials with regard to their specific behaviour in hydrogen applications.
To date, no limit has been defined for the permeability of the polymer base used in the compound. The technically relevant H2 permeation is investigated on material samples with standardised dimensions. Based on the permeability of the NBR polymer base compounds and taking into account the functional need for materials with higher hydrogen permeation resistance, Sattec has also developed new formulations with other polymer bases.
The graph shows the permeability values currently achieved with Sattec compounds. The test is carried out according to ZP 5101 at 23°C with 100% hydrogen.
Author:
Felice Pavan, SATTEC DBS gomma